MEDtube is the largest social eLearning platform for 350,000+ professionals sharing 30,000+ videos, courses, images, documents and webinars
Register for freeVitreous Blocked Glaucoma Shunt Tube
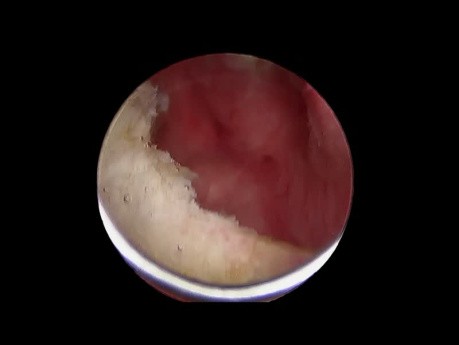
This video demonstrates a technique to clear vitreous from a sulcus placed glaucoma shunt device. A previously working device suddenly failed and was suspected of vireous incarceration. Vitrectomy with...
Laparoscopic Transabdominal Preperitoneal Left...
This video depicts the laparoscopic repair of an L4, left subcostal incisional hernia via a transabdominal preperitoneal approach.
Meniscal Knee Injuries
Meniscal knee injuries. Join the channel membership to unlock access to premium courses https://www.youtube. com/channel/UCpKGX6esbmV364XDaTQGckQ/join
Left Atrial Myxoma Embolism to the Descending...
Clinical video case: patient with the huge Lt atrial myxoma came with lower extremities ischemia and occlusion as a result of myxoma avulsion.
Post PTE and Cardiac Arrest Organ Donation
Professor Zeraatian's expertise in the field of organ donation extends to donation after cardiac death, where he has made significant contributions to advancing this critical area of medicine. Through...
TURP - Abscess of the Prostate
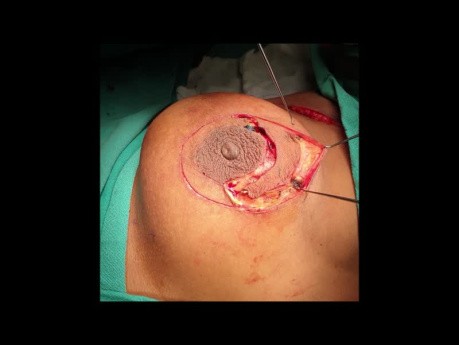
Urology video case: male, 72 years old, presented with urinary retention. Prostate 120 cm3 in ultrasound, PSA 11. RM - 40 cm3 abscess of the prostate.
Breast Oncoplasty: Different Volume Displacement...
Breast reconstruction following a mastectomy aims to restore the natural appearance of the breasts, helping survivors regain confidence, a sense of empowerment, and a sense of wholeness. Autologous tissue...
OrthoPioneers - John Charnley
OrthoPioneers - John Charnley. Join the channel membership to unlock access to premium courses https://www.youtube. com/channel/UCpKGX6esbmV364XDaTQGckQ/join
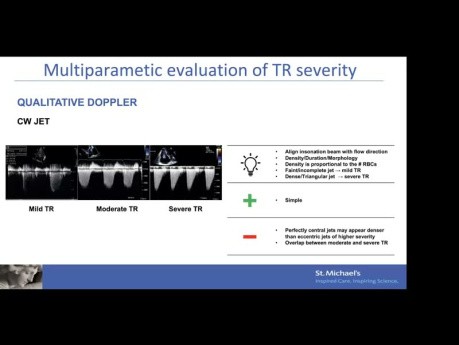
Echo Assessment of Tricuspid Regurgitation –...
Echo assessment of tricuspid regurgitation – mechanism, quantification and device selection. Speaker: Mathias Claeys MD PhD, Interventional Echocardiography Fellow, St. Michael's Hospital. Objectives:...
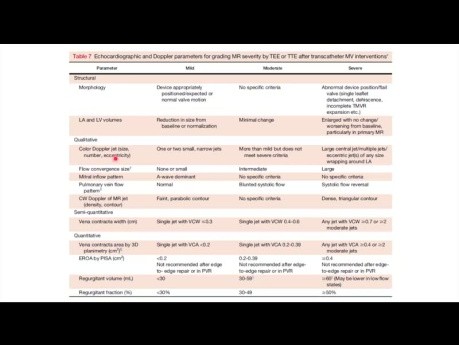
Case Potpourri: Regurgitation after Transcatheter...
Regurgitation after transcatheter edge-to-edge repair. Date: Thursday, 1 Feb 2024 Speaker: Flora Huang, MD FRCPC Echo Fellows, St. Michael’s Hospital. Objectives: 1. Review assessment of valvular...
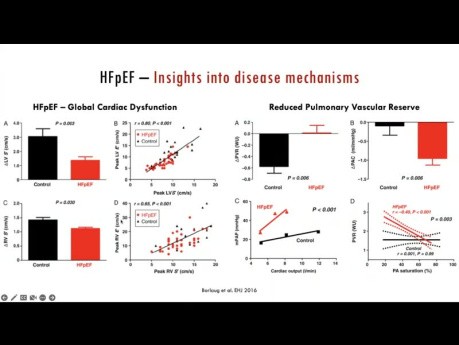
Stressing the Stepchild - Clinical Utility of...
Stressing the stepchild - Clinical Utility of Evaluating the RV-PV Unit During Exercise. Speaker: Mathias Claeys, MD PhD. Interventional Echocardiography Fellow, St. Michael's Hospital. Twitter:...
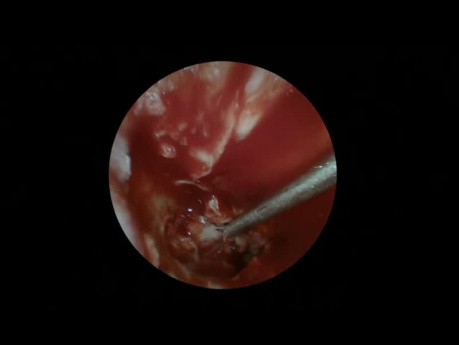
Chronic Suppurative Otitis Media Tympanoplasty
ENT video case: this is a demonstration of an endoscopic tympanoplasty as a treatment for chronic suppurative otitis media.
Featured channels
Mitral Valve Repair Center at The Mount...
As one of the world's most respected and experienced heart…
EUS - ENDO
EUS-ENDO will offer one day of live transmission from Paoli-Calmettes…
SAGES - Society of American Gastrointestinal...
The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) is…