MEDtube is the largest social eLearning platform for 350,000+ professionals sharing 30,000+ videos, courses, images, documents and webinars
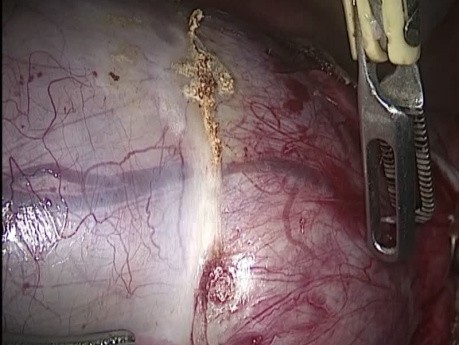
Register for freeHuge Teratoma Cystectomy
Laparoscopic cystectomy of huge ovarian teratoma, with monopolar and bipolar forceps with ovarian cortex preservation.
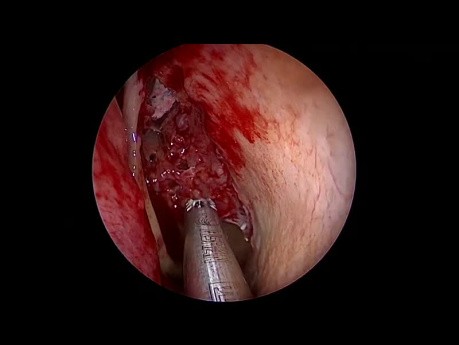
Complete Endoscopic Sinus Surgery - Systematic...
This is a demonstration of a complete endoscopic sinus surgery using the vertical lamellas concept, swing door technique and intact bulla technique to the frontal recess.
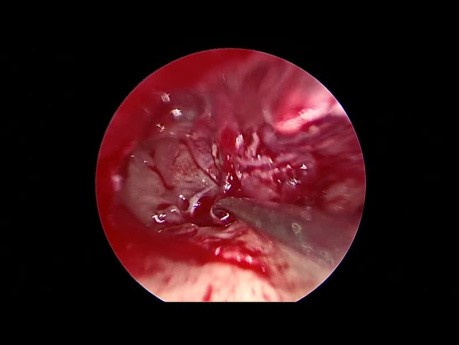
Endoscopic Revision Tympanoplasty for Conductive...
This is an edited video for an endoscopic revision tympanoplasty done to correct a postoperative conductive hearing loss. Audio on for commentaries on this endoscopic ear surgery video.
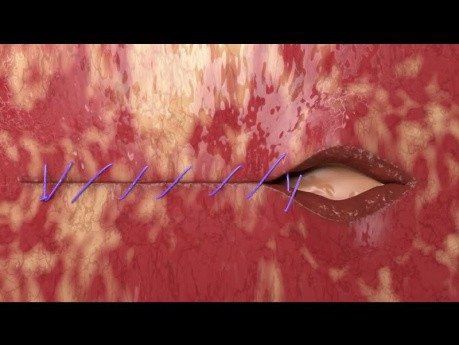
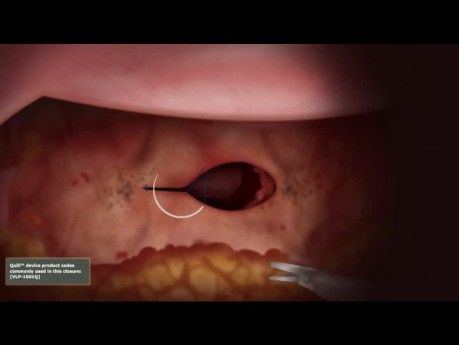
Vaginal Cuff Closure Unidirectional Quill® Barbed...
Vaginal Cuff closure with unidirectional Quill® barbed suture.
Laparoscopic Myomectomy Animation 3 Layer Closure...
Animation showing the correct use of Quill® barbed sutures during laparoscopic myomectomy.
What is a Stable Pelvic Ring Injury?
What is a stable pelvic ring injury? Join the channel membership to unlock access to premium courses.
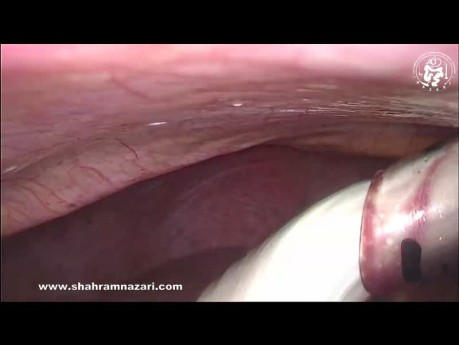
Hemostasis of Liver Bed in Laparoscopic Cholecystectomy
During laparoscopic cholecystectomy the most life threatening complication is haemorrhage. The well known methods to the achieve hemostasis from the gallbladder bed are; use of electro-cautery, (*brand...
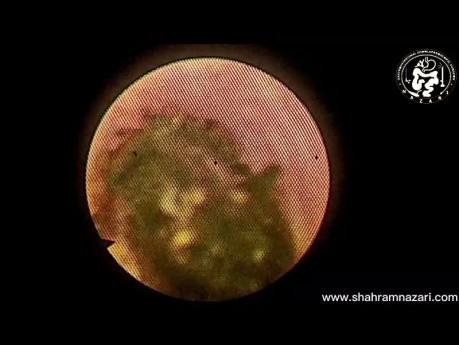
Hourglass Gallbladder Remnant Concomitant with...
Surgical video case: gallbladder anatomy is highly variable, and surgeons must be prepared to identify anomalies of form, number, and position. Variants include gallbladder agenesis, diverticulum, duplication,...
Proximal Humerus Reduction Parameters - OrthoConcepts
Proximal humerus reduction parameters - OrthoConcepts. Join the channel membership to unlock access to premium courses.
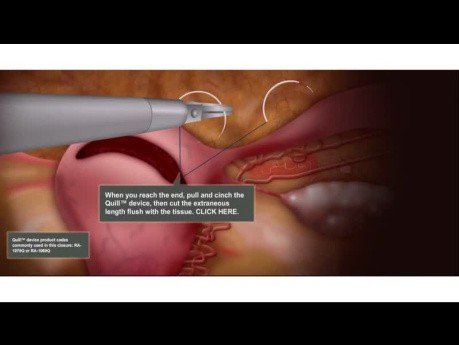
Laparoscopic Hysterectomy - Vaginal Cuff Closure...
Animation showing the correct use of Quill barbed sutures during laparoscopic hysterectomy.
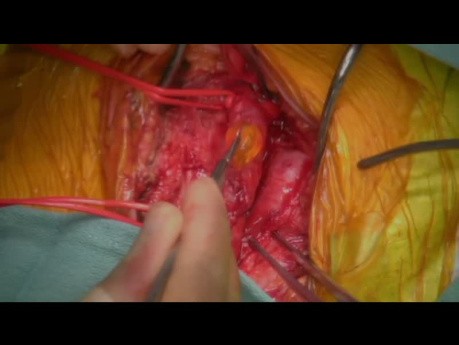
Left Atrial Myxoma Embolism to the Descending...
A left atrial myxoma is a rare type of cardiac tumor that originates in the left atrium of the heart. These tumors are typically benign and composed of a mix of connective tissue and cells. While often...
Proximal Humerus Radiographic Parameters
Orthopaedic video: proximal humerus radiographic parameters. Join the channel membership to unlock access to premium courses.
Featured channels
Mitral Valve Repair Center at The Mount...
As one of the world's most respected and experienced heart…
EUS - ENDO
EUS-ENDO will offer one day of live transmission from Paoli-Calmettes…
SAGES - Society of American Gastrointestinal...
The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) is…